Alfresa Pharma Corporation
| Name | Alfresa Pharma Corporation |
|---|---|
| Address | 2-2-9 Kokumachi, Chuo-ku, Osaka 540-8575 |
| Phone | +81-6-6941-0300 |
| FAX | +81-6-6947-1548 |
| URL | https://www.alfresa-pharma.co.jp/ |
| Contact | Sales of Commisioned Business TEL:81-276-52-6032 E-mail:info@alfresa-pharma.co.jp |
Our Strength
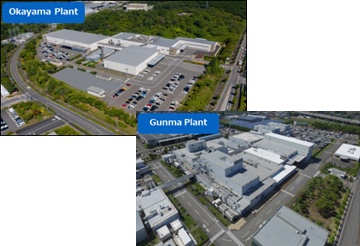
We are engaged in contract manufacturing of ethical and over-the-counter pharmaceuticals, quasi-drugs, and more at our Gunma and Okayama plants. In April 2023, the Commissioned Business Division was established to further strengthen the foundation of formulation development and contract manufacturing. Currently, we are conducting contract manufacturing of a wide variety of solid and liquid agents that meet various standards for pharmaceutical manufacturers, including foreign companies. We are also focusing on improving packaging technology, and are capable of special packaging such as strip packaging for solid dosage forms, and aseptic filling of liquid dosage forms from glass containers into PE containers. We are actively taking up the challenge to create new dosage forms and manufacturing methods.
Moreover, we are currently developing a contracting system for manufacturing of highly pharmacologically active formulations, we will position as a priority area for future business expansion.
■Extensive support from the early stages of development to regulatory approval
In addition to contract manufacturing, we offer a wide range of services from the initial stage of development, such as formulation development, investigational drug manufacturing, and analytical method development, to the preparation of documents for application and support for obtaining regulatory approval. We adapt flexibly to meet our client’s needs.
■Integrated product supply support from manufacturing to sales (Total supply chain service)
We have development a system that allows us to single-handedly undertake the wide variety of operations necessary for the supply of pharmaceutical products through the collaboration of Alfresa Group companies. In addition, by utilizing the Alfresa Group’s network, we deliver high-quality pharmaceuticals and services to our customers with safety, reliability, and sincerity through our integrated total supply chain service that covers everything from manufacturing to sales.